Projekte
3D Human Tissue-Engineered Models for Drug Discovery
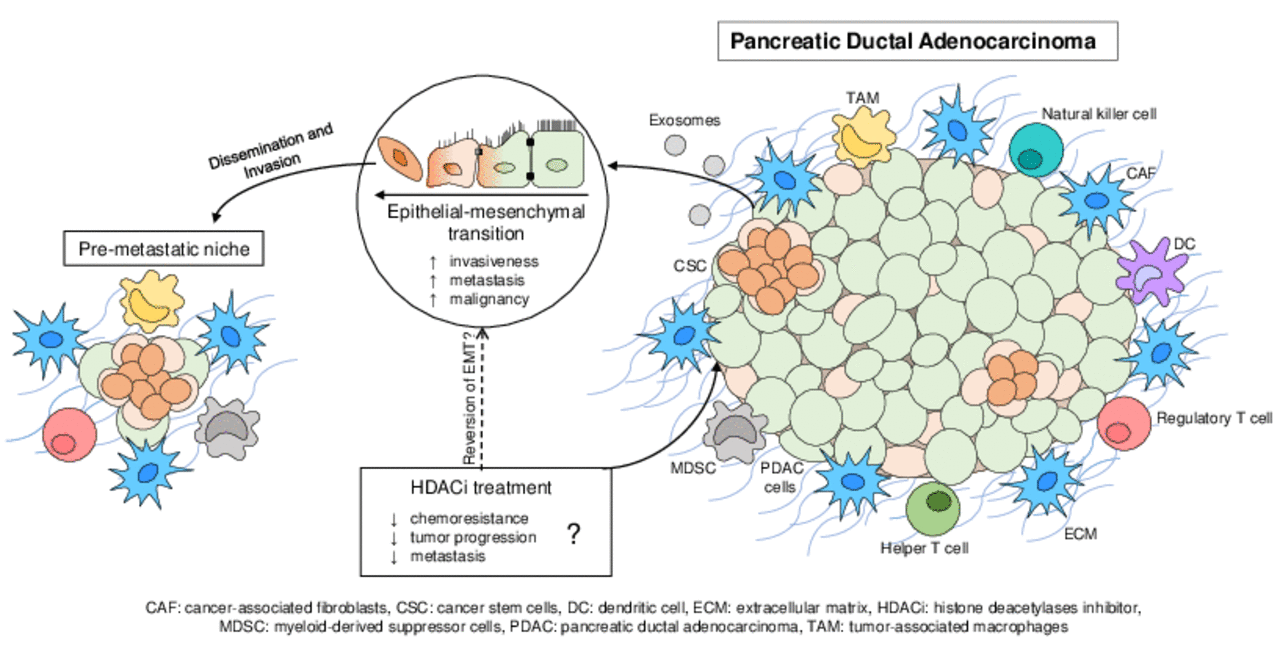
Targeting the tumor microenvironment of pancreatic cancer

Pancreatic Ductal Adenocarcinoma (PDAC), a highly aggressive form of pancreatic cancer, is known for displaying early metastasis and enhanced resistance against classical chemotherapeutic treatment regiments, leading to a very low overall survival rate. This troubling and detrimental pathophysiological feature, impeding and exacerbating patient’s treatment, is mainly caused by the characteristic fibrotic desmoplasia of the tumor microenvironment (TME), which mainly consists of so-called cancer-associated fibroblasts (CAF), extensive amounts of extracellular matrix and various other recruited types of tumor‑promoting cells.
In our research project, we established a 3D spheroid co-culture model using PDAC tumor cells and patient-derived cancer-associated fibroblasts to investigate the biological interactions and to analyse the efficacy and impact of novel drug candidates. A potential and promising therapeutical target is the significant cellular process of epithelial-mesenchymal transition (EMT), in which immobile tumor cells acquire migratory properties associated with increased malignancy, invasiveness and metastasis activity. The reversion of malignant tumor cells to their epithelial state is theorized to diminish chemoresistance and metastasis significantly, thereby improving the chemotherapeutic intervention and treatment. Further research includes an extension of our 3D spheroid co-culture model to include endothelial and immune cells, and a 3D tumor microenvironment-on-chip model for personalized drug testing.
Selected publications:
- Reimche I, Yu H, Ariantari NP, Liu Z, Merkens K, Rotfuß S, Peter K, Jungwirth U, Bauer N, Kiefer F, Neudörfl JM, Schmalz HG, Proksch P, Teusch N, Phenanthroindolizidine Alkaloids Isolated from Tylophora ovata as Potent Inhibitors of Inflammation, Spheroid Growth, and Invasion of Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 2022, 23(18), 10319. DOI: 10.3390/ijms231810319
- Xie B, Hänsel J, Mundorf V, Betz J, Reimche I, Erkan M, Büdeyri I, Gesell A, Kerr RG, Ariantari NP, Yu H, Teusch N, Mrsny RJ, Pseudopterosin and O-Methyltylophorinidine Suppress Cell Growth in a 3D Spheroid Co-Culture Model of Pancreatic Ductal Adenocarcinoma. Bioengineering, 2020, 7 (2), 57. DOI: 10.3390/bioengineering7020057
- Movahhed S, Westphal J, Kempa A, Schumacher C E, Sperlich J, Neudörfl J-M, Teusch N, Hochgürtel M, Schmalz H-G, Total Synthesis of (+)‐Erogorgiaene and the Pseudopterosin A−F Aglycone via Enantioselective Cobalt‐Catalyzed Hydrovinylation, Chemistry – A European
Pharmacological and toxicological compound assessment in a novel 3D immune competent skin model
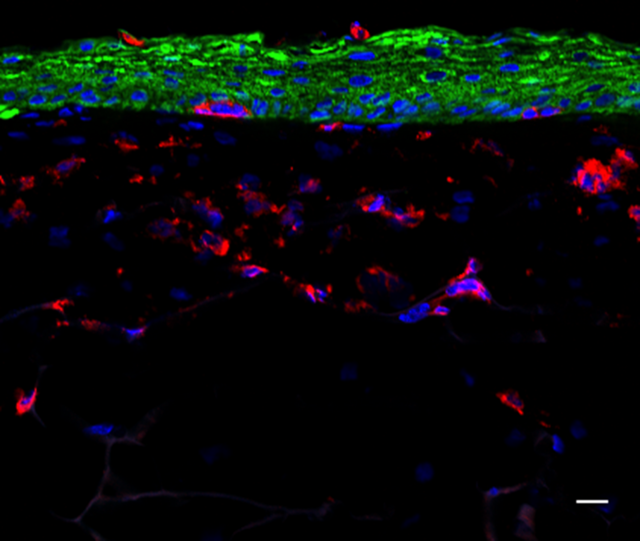
As the largest organ of the body, the skin is permanently exposed to environmental hazards, radiation, chemical substances and biological agents, thereby forming the first line of immunological defense in the body. However, these factors can lead to serious skin reactions such as skin irritation, inflammation, allergies or cancer. Hence, there is a substantial need to screen and assess the toxicity of agents and the identification of efficacious novel drug candidates for the skin.
We are currently developing a novel human 3D immune competent full-thickness skin model according to the 3R principles (“replace”, “reduce” and “refine”), for pharmacological and toxicological compound assessment. For this, langerhans cell surrogates are integrated as central initiators of the immune reaction in the skin, implementing skin sensitization and inflammation in the 3D model. To assess the tested compounds, inflammatory pathways are investigated via sequencing and transcriptomic analysis and threshold values will be determined for putative compound classifications and perspectively for high throughput screenings.
Further research includes the integration of additional cell types e.g. melanocytes, melanoma cells or vascularization, allowing distinct disease modelling for drug discovery.
Natural Product Bioactivity-Guided Screening


Since antiquity, nature has served as a source of remedies for many diseases of mankind. Notwithstanding the development of many modern synthetic drugs, there is a huge need for exploring bioactive natural products which could serve as lead compounds for drug discovery and development. Although the early days of natural products’ discovery efforts focused on the isolation of interesting secondary metabolites from plants, sponges, soft or hard corals, …. etc, by time attention turned into smaller creatures, such as endophytic and marine fungi, which have proven themselves as rich sources of remarkable biologically active secondary metabolites. One of the undergoing research projects conducted in our group comprises the study of fungi from different habitats (terrestrial and marine habitats) as potential producers of new bioactive natural products. The workflow starts with collecting samples followed by isolation and purification of several fungi. The isolated fungi are then identified and cultivated on suitable culture media. The obtained crude extracts are then tested against several bioassays in a bioactivity-guided approach. Afterwards, the biologically active extracts are fractionated via different chromatographic techniques including column chromatography utilizing different stationary phases, liquid chromatography and HPLC (High Performance Liquid Chromatography) till single pure compounds are obtained. The structures of isolated compounds are deduced based on a combination of 1D and 2D NMR spectroscopy and HRESIMS data as well as by comparison with the literature. For full characterization of compounds, absolute configurations should be assigned based on optical rotations (OR) measurements and comparison with the literature values, modified Mosher’s method (MMM), electronic circular dichroism (ECD) calculations or single-crystal X-ray diffraction analysis (XRD). The last step includes the evaluation of different bioactivities of the isolated structurally characterized compounds including cytotoxicity against different human cell lines, anti-inflammatory activity and many other activities.
Selected publications:
- Reimche I, Yu H, Ariantari NP, Liu Z, Merkens K, Rotfuß S, Peter K, Jungwirth U, Bauer N, Kiefer F, Neudörfl JM, Schmalz HG, Proksch P, Teusch N, Phenanthroindolizidine Alkaloids Isolated from Tylophora ovata as Potent Inhibitors of Inflammation, Spheroid Growth, and Invasion of Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 2022, 23(18), 10319. DOI: DOI: 10.3390/ijms231810319
- El-Kashef DH, Youssef FS, Reimche I, Teusch N, Müller WEG, Lin W, Frank M, Liu Z, Proksch P, Polyketides from the marine-derived fungus Aspergillus falconensis: In silico and in vitro cytotoxicity studies. Bioorganic & Medicinal Chemistry, 2021, 29, 115883. DOI: 10.1016/j.bmc.2020.115883
- El-Kashef DH, Youssef FS, Hartmann R, Knedel TO, Janiak C, Lin W, Reimche I, Teusch N, Liu Z, Proksch P, Azaphilones from the Red Sea Fungus Aspergillus falconensis. Marine Drugs, 2020, 18 (4), 204. DOI: 10.3390/md18040204
- Xie B, Hänsel J, Mundorf V, Betz J, Reimche I, Erkan M, Büdeyri I, Gesell A, Kerr RG, Ariantari NP, Yu H, Teusch N, Mrsny RJ, Pseudopterosin and O-Methyltylophorinidine Suppress Cell Growth in a 3D Spheroid Co-Culture Model of Pancreatic Ductal Adenocarcinoma. Bioengineering, 2020, 7 (2), 57. DOI: 10.3390/bioengineering7020057
- Yu H, Sperlich J, Höfert SP, Janiak C, Teusch N, Stuhldreier F, Wesselborg S, Wang C, Kassack M, Dai H, Liu Z, Proksch P, Azaphilone pigments and macrodiolides from the coprophilous fungus Coniella fragariae. Fitoterapia, 2019, 137, 104249. DOI: 10.1016/j.fitote.2019.104249

