Working group Kalscheuer
News
Contribution of the Kalscheuer lab to a seminal study on the BacPROTAC concept published in the leading life science journal Cell
03.05.2023
The Kalscheuer lab is pleased to share a contribution to a seminal study reporting on development of the BacPROTAC concept as a new antimycobacterial treatment option published in the leading life science journal Cell (IF 66.85). This study was conducted in collaboration with Tim Clausen´s lab at the Vienna BioCenter, Lukas Junk at Saarland University, and further collaegues.
Mycobacterium tuberculosis, the causative agent of tuberculosis, was the second leading cause of death by a single pathogen after SARS-CoV-2 in 2021. Although the Corona pandemic has been overcome, tuberculosis remains a major global health burden. Discovering new effective drugs to tackle the increasing problem of emerging multi-drug resistant tuberculosis proves to be difficult due to the high intrinsic resistance of M. tuberculosis.
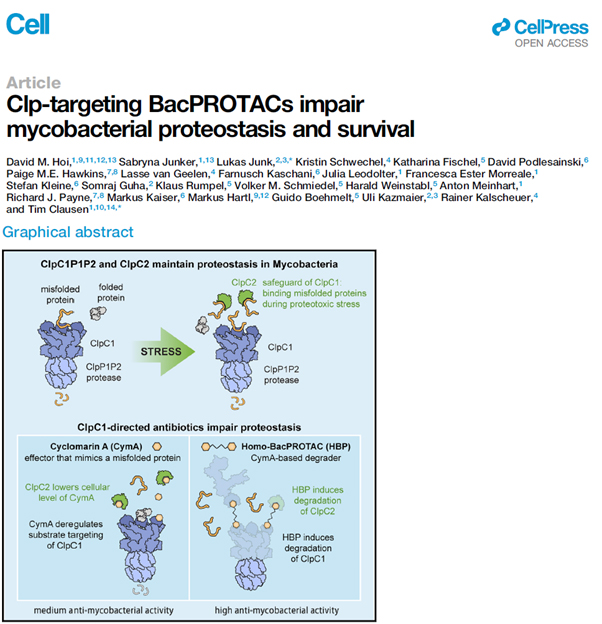
A promising drug target is the Clp system in M. tuberculosis, which is essential to eliminate misfolded and futile proteins. An increase or decrease in the Clp activity could lead to an imbalance of protein synthesis and salvage ultimately resulting in cell death. The natural products cyclomarin A and ecumicin can bind to one crucial component of the Clp system, the unfoldase ClpC1, which prevents ClpC1 to interact normally with misfolded or futile proteins. As a result, the Clp system transitions into hyperactivity and therefore degrades nearby proteins, which might be important for cell survival. However, the bacterial defence towards cyclomarin A and ecumicin reacts in upregulation of another Clp protein, ClpC2. This protein protects the ClpC1 protein from binding cyclomarin A and ecumicin and decreases the efficiency of the drugs.
Homo-BacPROTACs, which have been designed and evaluated in this study, consist of two cyclomarin A-molecules, which both can bind separate ClpC1 proteins. Binding two different ClpC1 proteins brings them in close proximity to one another resulting in degradation and impaired activity of the Clp system. Additionally, the Homo-BacPROTACs also target ClpC2 and thereby circumvent the resistance mechanism. The BacPROTAC system can be altered and adapted to target other proteins and selectively degrade these proteins facilitating the Clp system. This highly adaptive and innovative technology provides a new way to tackle the rising problem of antimicrobial resistance in M. tuberculosis.
Original publication:
David M. Hoi*, Sabryna Junker*, Lukas Junk, Kristin Schwechel, Katharina Fischel, David Podlesainski, Paige M. E. Hawkins, Lasse van Geelen, Farnusch Kaschani, Julia Leodolter, Francesca Ester Morreale, Stefan Kleine, Somraj Guha, Klaus Rumpel, Volker M. Schmiedel, Harald Weinstabl, Anton Meinhart, Richard J. Payne, Markus Kaiser, Markus Hartl, Guido Boehmelt, Uli Kazmaier, Rainer Kalscheuer, Tim Clausen: “Clp-targeting BacPROTACs impair mycobacterial proteostasis and survival”. Cell (2023), DOI: 10.1016/j.cell.2023.04.009.
*These authors contributed equally.
________________________________________________________________
06.10.2022
The Kalscheuer lab congratulates Prof. Carolyn R. Bertozzi for being awarded the Nobel Prize in Chemistry 2022 "for the development of click chemistry and bioorthogonal chemistry" !
Check out our translational collaborative work with Carolyn using bioorthogonal chemistry to study and exploit trehalose metabolism in Mycobacterium tuberculosis.