Projects
The causative agent of pulmonary tuberculosis in humans, Mycobacterium tuberculosis, is the most significant bacterial pathogen. Despite decades of research, 1.5 million people continue to die annually from this poverty-related infectious disease. Despite decoding of the genome of Mycobacterium tuberculosis already in 1998, the function and relevance of a large number of genes has still not been elucidated. To make a contribution to the fight against the global tuberculosis pandemic, the Kalscheuer group is performing extensive molecular genetic manipulation of Mycobacterium tuberculosis to elucidate the significance of genes of unknown function. Using specialized transduction employing temperature-sensitive Mycobacteriophages, we produce gene deletion mutants, conditional mutants, merodiploid strains and overexpression strains to address specific questions. Hereby, essential genes and virulence factors will be identified and subsequently comprehensively characterized by complementary molecular biology and biochemistry approaches. The studied gene products may represent potential new antibiotic targets and allow rational structure-based drug design. Particularly, we are interested in genes involved in cell wall formation and in glycoconjugate biosynthesis in Mycobacterium tuberculosis.

Selected publications:
Babu Sait MR, Koliwer Brandl H, Stewart JA, Swarts BM, Jacobsen M, Ioerger TR, Kalscheuer R. PPE51 mediates uptake of trehalose across the mycomembrane of Mycobacterium tuberculosis. Sci Rep 12, 2097 (2022).
Kamariza M, Shieh P, Ealand CS, Peters JS, Chu B, Rodriguez-Rivera FP, Babu Sait MR, Treuren WV, Martinson N, Kalscheuer R, Kana BD, Bertozzi CR. Rapid detection of Mycobacterium tuberculosis in sputum with a solvatochromic trehalose probe. Sci Translat Med 10, eaam6310 (2018).
Korte J, Alber M, Trujillo CM, Syson K, Koliwer-Brandl H, Deenen R, Köhrer K, DeJesus MA, Hartman T, Jacobs WR Jr, Bornemann S, Ioerger TR, Ehrt S, Kalscheuer R. Trehalose-6-phosphate-mediated toxicity determines essentiality of OtsB2 in Mycobacterium tuberculosis in vitro and in mice. PLoS Pathog. 12:e1006043 (2016).
Koliwer-Brandl H, Syson K, van de Weerd R, Chandra G, Appelmelk B, Alber M, Ioerger TR, Jacobs Jr. WR, Geurtsen J, Bornemann S, Kalscheuer R. Metabolic network for the biosynthesis of intra- and extracellular α-glucans required for virulence of Mycobacterium tuberculosis. PLoS Pathog 12, e1005768 (2016).
Kalscheuer R, Syson K, Veeraraghavan U, Weinrick B, Biermann KE, Liu Z, Sacchettini JC, Besra G, Bornemann S, Jacobs WR, Jr. Self-poisoning of Mycobacterium tuberculosis by targeting GlgE in an alpha-glucan pathway. Nat Chem Biol 6, 376-384 (2010).
Kalscheuer R, Weinrick B, Veeraraghavan U, Besra GS, Jacobs WR, Jr. Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 107, 21761-21766 (2010).
The pharmacological potential of natural substances has been known even before the discovery of penicillin and its antibacterial properties in 1928. Since then, a large number of compounds that are now indispensable in medicine have been isolated from bacteria and microbial fungi in particular. The discovery of new and known natural products of microbial origin with a focus on undescribed antimicrobial bioactivities is therefore one of our fundamental goals in order to contribute to the increasingly urgent fight against a worsening microbial resistance situation.
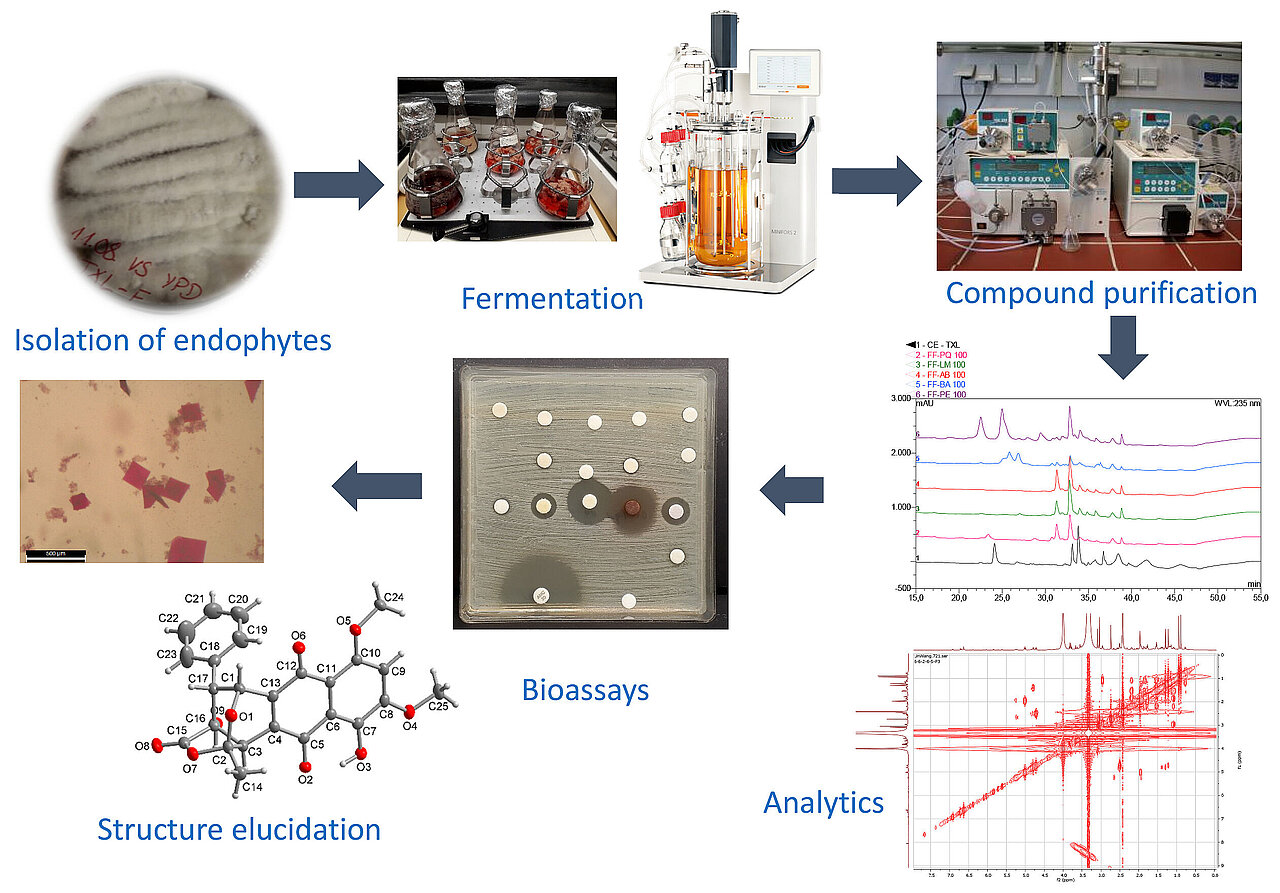
Our typical workflow starts with the isolation of microorganisms from diverse environmental samples or of endophytic fungi and bacteria from plant (parts). Solvent extracts obtained from biomass and culture supernatants of these isolates are then tested for antimicrobial activities against a broad spectrum of human pathogenic microorganisms. Purification of crude extracts to isolated pure compounds is bioactivity-guided via various preparative methods such as liquid-liquid extraction, VLC (vacuum liquid chromatography), Sephadex chromatography, RP-VLC (reverse-phase vacuum liquid chromatography) or semi-preparative HPLC. The obtained pure compounds are then structurally elucidated via various analytical methods, such as MS (mass spectrometry), one- and two-dimensional NMR (nuclear magnetic resonance), and the acquisition of a UV-Vis spectrum. More detailed stereochemical elucidation can be achieved via specific NMR techniques, polarimetry, chemical derivatization reactions, X-ray crystallographic imaging, and measurement of circular dichroism. New active compounds and known compounds with undescribed activity are subsequently screened and characterized for their antimicrobial and cytotoxic potential in more detailed antimicrobial and cytotoxic assays.
Selected publications:
Eze PM, Simons V, Seidemann T, Wang L, Kiffe-Delf AL, Frank M, van Geelen L, Abba CC, Esimone CO, Okoye FBC, Kalscheuer R. Serratiochelins A and B from Serratia marcescens show xenosiderophoric characteristics towards Acinetobacter baumannii and Mycobacterium tuberculosis. Trop J Pharm Res 20, 2551-2658 (2021).
Eze P, Gao Y, Liu Y, van Geelen L, Ejikeugwu C, Esimone C, Okoye F, Proksch P, Kalscheuer R. Secondary metabolites of a marine-derived Penicillium ochrochloron. Not Sci Biol 13, 11020 (2021).
The rapid increase in antimicrobial resistance is a growing problem worldwide and is also known as the antimicrobial resistance crisis (AMR crisis). This is not only a burden for medical care, but also poses economic challenges for many countries. The World Health Organization (WHO) reacted a few years ago and compiled a list of pathogens for which there will be few effective therapies soon unless the use of antibiotics changes drastically and the spread of resistance at the current pace will be stopped. These bacteria are summarized under the apronym ESKAPE and include both Gram-positive (Enterococcus faecium and Staphylococcus aureus) and Gram-negative human pathogenic bacteria (Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.).
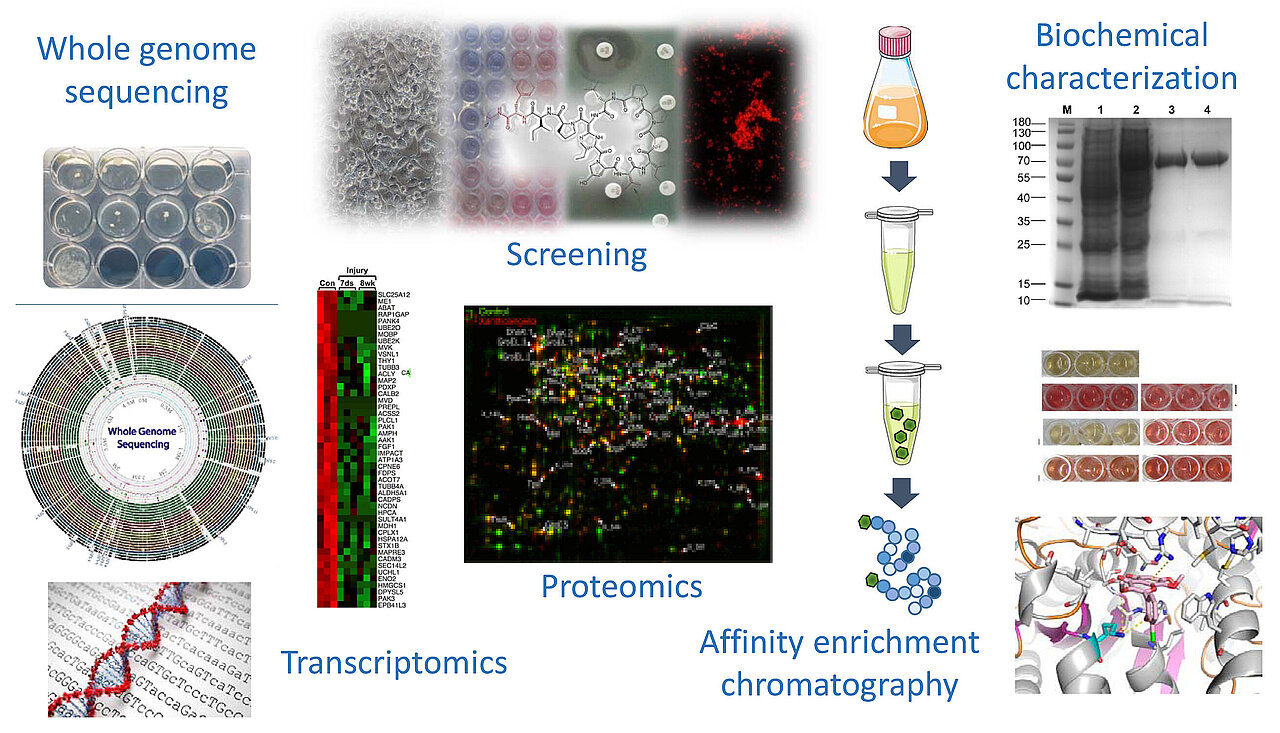
Our typical workflow begins with screening of both isolated natural products and (semi-)synthetic molecules for their antimicrobial potential. In addition to the ESKAPE pathogens, fungi such as Candida albicans as well as Mycobacterium tuberculosis, the causative agent of pulmonary tuberculosis, are also included in our routine screening efforts. Various human cell lines are subsequently employed to determine the cytotoxic properties of the active molecules in order to identify antimicrobial substances exhibting a favorable therapeutic window. Complementary methods such as the generation of spontaneously resistant mutants, genome sequencing, killing kinetics, transcriptome and proteome analysis of the stress response profile elicited in compound-treated cells as well as various biochemical and genetic methods are used to further characterize the antimicrobial properties of the most promising molecules and to elucidate their mechanism of action and resistance. This work shall contribute to countering the AMR crisis and to developing new effective therapeutics for the treatment of infections caused by multidrug-resistant pathogens.
Selected references:
Rehberg N, Sommer G, Drießen D, Kruppa M, Adeniyi ET, Chen S, Wang L, Wolf K, Tasch B, Ioerger TR, Zhu K, Müller TJJ, Kalscheuer R. Nature-inspired (di)azine-bridged bisindole alkaloids with potent antibacterial in vitro and in vivo efficacy against Methicillin-resistant Staphylococcus aureus. J Med Chem 63, 12623-12641 (2020).
van Geelen L, Kaschani F, Sazzadeh SS, Adeniyi ET, Meier D, Proksch P, Pfeffer K, Kaiser M, Ioerger TR, Kalscheuer R. Natural brominated phenoxyphenols kill persistent and biofilm-incorporated cells of MRSA and other pathogenic bacteria. Appl Microbiol Biotechnol 104, 5985-5998 (2020).
Meier D, Vázquez Hernández M, van Geelen L, Muharini R, Proksch P, Bandow JE, Kalscheuer R. The plant-derived chalcone Xanthoangelol targets the membrane of Gram-positive bacteria. Bioorg Med Chem 27, 115151 (2019).
Rehberg N, Omeje E, Ebada SS, van Geelen L, Liu Z, Sureechatchayan P, Kassack MU, Ioerger TR, Proksch P, Kalscheuer R. 3-O-Methyl-alkylgallates inhibit fatty acid desaturation in Mycobacterium tuberculosis. Antimicrob Agents Chemother 63, e00136-19 (2019).
Rehberg N, Akone HS, Ioerger TR, Erlenkamp G, Daletos G, Gohlke H, Proksch P, Kalscheuer R. Chlorflavonin targets acetohydroxyacid synthase catalytic subunit IlvB1 for synergistic killing of Mycobacterium tuberculosis. ACS Infect Dis. 4, 123-134 (2018).

