Institute of Pharmaceutical Biology and Biotechnology
Institute of Pharmaceutical Biology and Biotechnology
Welcome on the website of the Institute of Pharmaceutical Biology and Biotechnology at the Heinrich Heine University Düsseldorf.

News
Contribution of the Kalscheuer lab to a study advancing the homo-BacPROTAC concept as new antimycobacterial treatment option published in Nature Communications

05.03.2024
See our recent contribution to a study advancing the homo-BacPROTAC concept as new antimybacterial treatment option published in Nature Communications. This study was conducted in collaboration with Guido Boehmelt and coworkers at Boehringer Ingelheim RCV, Vienna, Austria, Lukas Junk at Saarland University, and further colleagues.
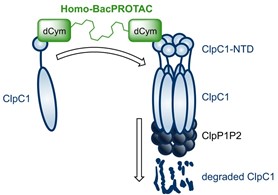
Homo-BacPROTACs consist of two cyclomarin A-molecules, which both can bind separate ClpC1 proteins. ClpC1 represents the mycobacterial ATP-driven unfoldase of the quasi-proteasomal like ClpC:ClpP (ClpCP) protease. Homo-BacPROTACs induce proximity between separate ClpC1 proteins, which results in selfdegradation of ClpC1 and impairs activity of the Clp protein quality control system. This innovative technology may provide a new way to tackle the rising problem of antimicrobial resistance in the human pathogen Mycobacterium tuberculosis.
Original publication:
Junk L*, Schmiedel VM*, Guha S, Fischel K, Greb P, Vill K, Krisilia V, van Geelen L, Rumpel K, Kaur P, Krishnamurthy RV, Narayanan S, Shandil RK, Singh M, Kofink C, Mantoulidis A, Biber P, Gmaschitz G, Kazmaier U, Meinhart A, Leodolter J, Hoi D, Junker S, Morreale FE, Clausen T, Kalscheuer R, Weinstabl H, Boehmelt G. Homo-BacPROTAC-induced degradation of ClpC1 as a strategy against drug-resistant mycobacteria. Nat Commun 15, 2005 (2024). https://doi.org/10.1038/s41467-024-46218-7
*These authors contributed equally.
________________________________________________________________

